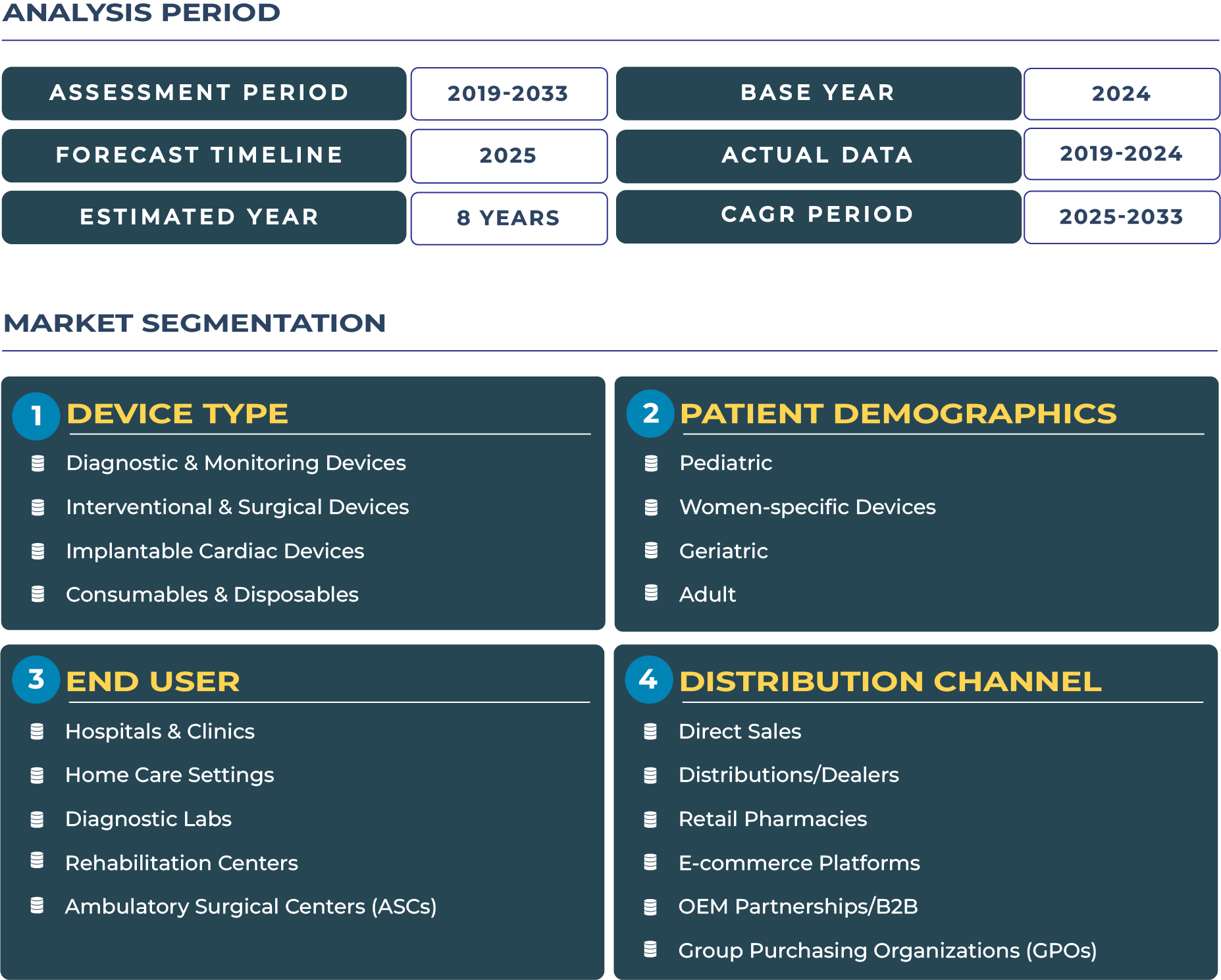
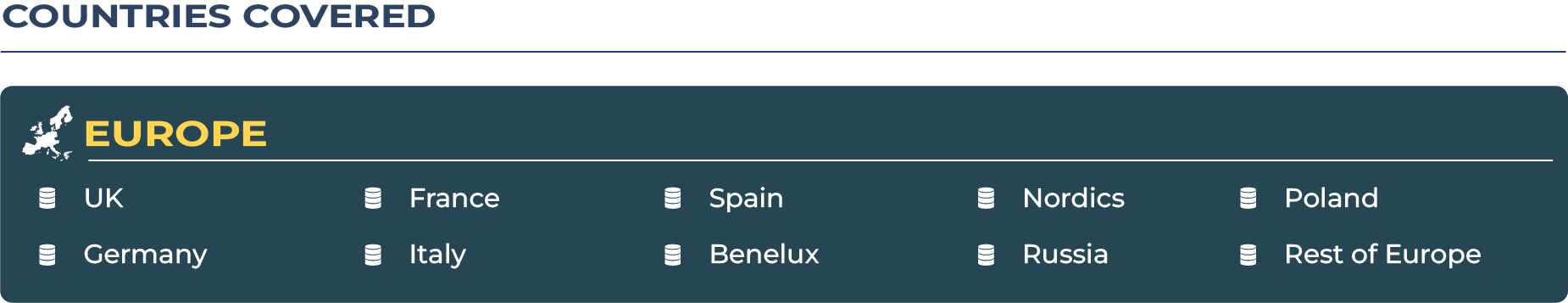
Europe Cardiovascular Devices Market: Navigating Harmonization, Innovation, and Regulation
Frame Europe as a harmonized market where MDR regulations and innovation hubs drive cardiovascular device evolution. The continent offers a unique blend of strict regulatory oversight, strong public-private collaboration, and advanced research ecosystems, which collectively shape the cardiovascular devices industry. Countries across Europe—from Germany and France to Poland and the Czech Republic—are modernizing their healthcare systems to manage the rising burden of cardiovascular disease, one of the leading causes of mortality in the region. Within this cardiovascular devices ecosystem, the balance of stringent regulation and high innovation capacity sets Europe apart as both a complex and opportunity-rich marketplace.
Europe Cardiovascular Devices Market Outlook: Harmonized Regulations and Innovation Ecosystems Driving Europe Cardiovascular Devices Sector
The Europe cardiovascular devices market is projected to expand from USD 14.48 billion in 2025 to USD 21.29 billion by 2033, registering a CAGR of 4.9% during 2025–2033. This steady growth trajectory reflects the combined impact of aging populations, expanding hospital infrastructure, and rising adoption of advanced cardiac procedures across Western and Eastern Europe. As national healthcare systems prioritize early detection and intervention, diagnostic and monitoring devices such as portable ECGs, wearable cardiac monitors, and imaging systems are witnessing strong uptake. Meanwhile, interventional and surgical devices—particularly catheters, stents, and minimally invasive surgical tools—are increasingly utilized to reduce patient recovery times and optimize outcomes.
The implementation of the EU Medical Device Regulation (MDR) is reshaping market dynamics by raising compliance costs but also ensuring stronger patient safety and market transparency. Innovation hubs in cities such as Amsterdam, Berlin, and Stockholm are at the forefront of developing personalized cardiac implants and hybrid surgical solutions. Additionally, cross-border EU funding programs, including Horizon Europe, are driving research collaboration, creating new opportunities for cardiovascular device development and commercialization. Despite geopolitical uncertainties and economic pressures, the cardiovascular devices landscape in Europe is anchored in a robust framework of regulation, innovation, and regional cooperation.
Drivers & Restraints: Key Growth Catalysts and Persistent Market Barriers in Europe Cardiovascular Devices Industry
Aging Populations, EU Funding, and Advanced Infrastructure Driving Adoption
Europe’s demographic profile remains one of the strongest growth drivers for the cardiovascular devices sector. According to the Eurostat, over 21% of the EU’s population is aged 65 and above as of 2024, a factor that significantly elevates demand for implantable cardiac devices and interventional tools. Advanced hospital networks across Germany, France, and the Nordics are investing in state-of-the-art cardiac units equipped with hybrid operating theaters, enabling both surgical and catheter-based interventions. EU-level initiatives, including EU4Health, are allocating billions of euros to strengthen cardiovascular care infrastructure. Together, these elements reinforce Europe as a highly structured but innovation-driven cardiovascular devices market.
Price Controls, Trade Barriers, and Reimbursement Delays Restricting Market Expansion
Despite strong demand, several structural restraints hinder accelerated market growth. National price control measures across Southern and Eastern Europe often limit profit margins for manufacturers, while delayed reimbursement cycles discourage faster adoption of high-cost implantable devices. The aftermath of Brexit has introduced trade complexities, impacting cross-border supply chains and raising costs for companies operating in both the UK and EU. Moreover, the cost of MDR compliance has disproportionately affected small and mid-sized enterprises, restricting their ability to bring innovative cardiovascular devices to market quickly. These challenges underscore the need for streamlined reimbursement frameworks and stronger harmonization of regulatory processes across EU member states.
Trends & Opportunities: Digital Health Integration and Cross-Border Collaboration Defining the Next Decade
Digital Platforms, Hybrid Surgeries, and Personalized Implants Transforming Care
Digital integration is revolutionizing the European cardiovascular devices ecosystem. Hospitals in Western Europe are deploying AI-driven diagnostic tools for early detection of arrhythmias, while telemonitoring platforms are extending care to remote regions. Hybrid surgery models—combining traditional open procedures with catheter-based techniques—are gaining traction in France, Italy, and the Benelux, reducing hospital stays and improving patient outcomes. Personalized cardiac implants, enabled by 3D printing and genomics-based design, are emerging as a key innovation focus in Germany and Scandinavia, reflecting the region’s leadership in precision medicine.
Cross-Border Healthcare, Local Manufacturing, and AI-Driven Trials Offering Growth Potential
Europe’s unique regulatory and trade framework opens substantial opportunities for cross-border healthcare. Patients are increasingly traveling within the EU to access advanced cardiovascular interventions in centers of excellence. Localized device manufacturing is also expanding, with countries like Poland and Hungary positioning themselves as cost-effective production hubs for consumables and disposables. Meanwhile, AI-based clinical trials, supported by EU research funds, are accelerating the validation of new cardiovascular technologies. Together, these opportunities underscore Europe’s role as both a sophisticated demand center and a global innovation hub for cardiovascular devices.
Regional Analysis by Country
Western Europe
Western Europe dominates the cardiovascular devices market due to its advanced healthcare infrastructure, high per capita healthcare spending, and early adoption of innovative cardiac technologies. Countries like Germany, France, and the UK are at the forefront of hybrid surgical adoption, AI-integrated cardiac monitoring, and widespread use of implantable devices. Strong reimbursement frameworks and EU-driven harmonization further support steady market growth in this sub-region.
Eastern Europe
Eastern Europe is emerging as a promising growth frontier for cardiovascular devices, driven by investments in hospital modernization and government-backed healthcare reforms. While affordability and reimbursement delays remain challenges, countries like Poland, Czech Republic, and Hungary are improving access to diagnostic and surgical cardiovascular solutions. Cross-border patient inflows and increasing localized manufacturing make this sub-region attractive for future growth.
Competitive Landscape: Strategic Alliances and Innovation Shaping Europe’s Cardiovascular Devices Sector
The competitive landscape in Europe is shaped by a mix of global leaders and regional innovators. Companies such as Philips, Medtronic, and Abbott remain at the forefront, supported by European innovation hubs. In 2023, the EU launched the EU4Health program, channeling significant funds into cardiovascular health initiatives. In 2022, Philips expanded its European cardiac R&D capabilities, while Abbott received CE Mark approval for its AVEIR leadless pacemaker, reflecting Europe’s leadership in regulatory acceptance and innovation. Strategies such as pan-EU partnerships, cost optimization, and sustainability programs are increasingly central to success. The cardiovascular devices landscape in Europe is therefore evolving around both compliance and creativity, creating opportunities for long-term market consolidation.