Innovation-Led Diagnostic Modernization Reshaping the North America Diagnostic Imaging Devices Market
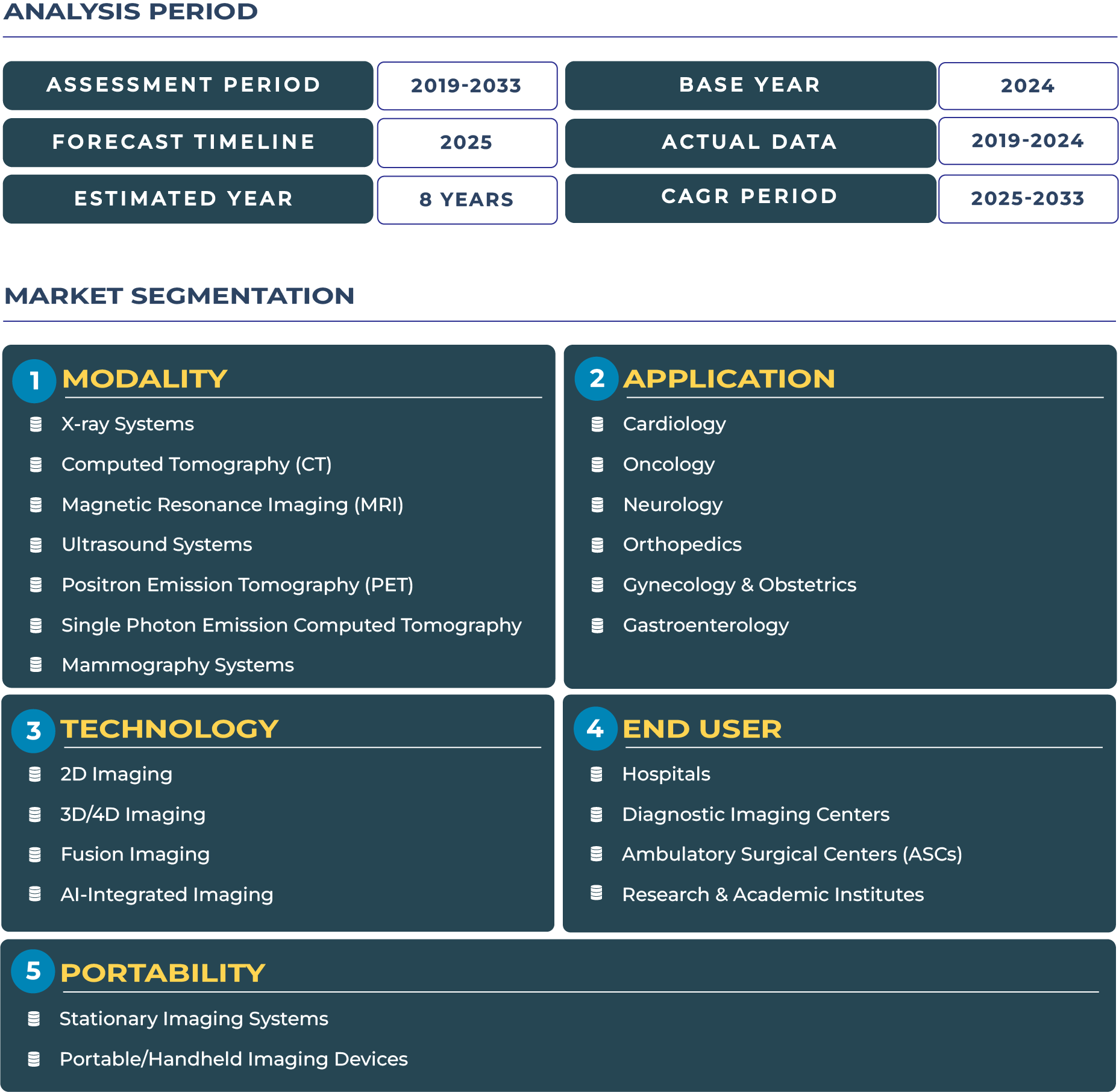
The North America Diagnostic Imaging Devices Market is undergoing a profound transformation as healthcare systems embrace AI-driven diagnostics, digital imaging workflows, and cloud-based collaboration. In 2025, the market is valued at USD 11.74 billion and is projected to reach USD 15.18 billion by 2033, expanding at a CAGR of 3.3%. This growth underscores how hospitals, imaging centers, and outpatient facilities are modernizing to align with precision medicine and value-based healthcare imperatives. The convergence of artificial intelligence, machine learning, and tele-imaging is transforming radiology into a data-driven ecosystem that supports earlier detection, optimized throughput, and predictive diagnostics across clinical settings.
Across the US, Canada, and Mexico, investments in digital transformation are accelerating as healthcare providers seek to replace aging MRI and CT systems with smart, AI-integrated modalities. Vendors are prioritizing interoperability and cloud storage to streamline image sharing between tertiary centers and rural clinics, addressing regional disparities in diagnostic access. North America’s leadership in research and regulatory innovation has fostered an environment where the integration of advanced modalities, such as photon-counting CT, portable ultrasound, and hybrid PET/MRI systems, is increasingly feasible at scale. These factors collectively position the region as the global hub for diagnostic imaging modernization.
North America Diagnostic Imaging Devices Market Outlook: Digital Integration Driving Sustainable Diagnostic Expansion
The North America Diagnostic Imaging Devices Market reflects a shift from hardware-centric procurement to digitally enabled, service-driven imaging ecosystems. The rise in chronic disease prevalence, aging populations, and demand for minimally invasive diagnostics has placed a premium on image-guided decision support. The US market, characterized by early adoption of cloud-based radiology information systems and picture archiving and communication systems, has set the benchmark for workflow automation. Canada and Mexico are also witnessing public-private investments in digital health infrastructure to standardize diagnostic delivery and address imaging backlog challenges intensified during the pandemic.
Market expansion is further supported by the introduction of AI software approved by the US Food and Drug Administration (FDA), facilitating automated lesion detection, triage, and workflow optimization. Increased collaboration between device manufacturers and healthcare institutions has accelerated the deployment of AI-powered image reconstruction technologies, enabling faster scan times and improved patient comfort. Furthermore, federal and provincial health initiatives in the region are encouraging investment in domestic manufacturing and sustainability in device supply chains, fostering long-term resilience in the diagnostic imaging ecosystem.
Drivers & Restraints: Balancing Modernization and Market Saturation
AI-Integrated Workflow Acceleration Fuels Growth Across Imaging Modalities
The primary driver of growth in the North America Diagnostic Imaging Devices Industry is the rapid commercialization of AI-assisted diagnostic platforms, which are transforming radiology from a reactive to a proactive specialty. Hospitals are accelerating capital refresh cycles for aging CT and MRI fleets to adopt systems capable of integrating seamlessly with AI algorithms that enhance diagnostic accuracy and throughput. Government-backed reimbursement models for digital imaging analytics and tele-imaging consultations have encouraged widespread adoption across tertiary centers. The expansion of portable ultrasound and handheld imaging solutions in point-of-care and emergency settings further reinforces the region’s focus on accessibility and clinical precision.
Procurement Challenges and Market Saturation Curtail Growth Momentum
Despite strong demand, high procurement costs and complex reimbursement procedures present formidable barriers to market expansion. In mature US urban markets, hospital networks already possess advanced imaging infrastructure, leading to slower unit sales growth. Smaller community hospitals face hurdles in accessing financing for advanced modalities such as PET/CT and photon-counting CT systems. Additionally, regulatory heterogeneity across states and provinces creates uncertainty in device adoption timelines. To mitigate these challenges, vendors are increasingly shifting toward subscription-based models and value-based procurement frameworks, aligning capital expenditure cycles with predictable operational outcomes.
Trends & Opportunities: Converging Digital Health and Imaging Ecosystems
From Hardware to Hybrid Services: The Rise of Imaging-as-a-Service
A prominent trend in the North American Diagnostic Imaging Devices Sector is the evolution from one-time equipment sales to long-term service contracts. Leading manufacturers are embracing SaaS-driven imaging analytics and predictive maintenance solutions that ensure continuous uptime and lifecycle value. Hospitals are integrating AI-enabled dashboards that provide operational insights on scan utilization, patient throughput, and energy consumption. This transition is redefining competitive differentiation, encouraging vendors to build partnerships around data intelligence rather than hardware alone. Additionally, radiology networks are leveraging AI to standardize interpretation and reporting across multi-site hospital systems.
Expanding the Reach of Portable and Point-of-Care Imaging
An equally significant opportunity lies in the proliferation of portable and handheld imaging systems across emergency and ambulatory care settings. The demand for immediate diagnostics in trauma, remote care, and mobile clinics has led to the widespread deployment of wireless ultrasound and compact CT systems. Governments and private providers in rural North America are collaborating to expand tele-imaging access, bridging the urban-rural diagnostic gap. As healthcare systems embrace decentralized care, the integration of mobile imaging solutions is poised to become a central pillar of regional healthcare delivery, particularly for underserved communities.
Regional Analysis by Country
-
United States
The US diagnostic imaging devices Market remains the global leader, driven by continuous investments in AI-enabled imaging and clinical decision support tools. Hospitals are increasingly adopting photon-counting CT and low-field MRI for advanced applications in oncology and neurology. -
Canada
Canada diagnostic imaging devices sector is focusing on interoperability and cross-province data integration. Federal initiatives are funding digitization programs to reduce diagnostic wait times and improve patient outcomes across public health systems. -
Mexico
Mexico diagnostic imaging devices market is expanding through public-private collaborations aimed at upgrading imaging capacity in secondary and tertiary hospitals. Localization of equipment maintenance and training programs has boosted efficiency and reduced operational costs.
Competitive Landscape: Strategic Collaborations Shaping the Diagnostic Imaging Future
The North America Diagnostic Imaging Devices Landscape is increasingly defined by cross-sector collaboration. In October 2025, CIVIE and North Star Diagnostic Imaging announced a partnership to modernize radiology services across 14 North Texas facilities, deploying unified RIS and billing integration to enhance operational efficiency. In August 2025, Esaote North America and Epica International entered a strategic agreement to expand MRI and CT solutions across both human and veterinary markets, demonstrating the region’s diversification strategy and service innovation focus.
Manufacturers are prioritizing hybrid business models that combine hardware sales with SaaS-based image management and analytics services, ensuring recurring revenue and improved customer retention. Continuous regulatory approvals from the FDA and strategic partnerships between technology companies and healthcare networks are accelerating the adoption of photon-counting CT, hybrid PET/MRI, and AI-enabled ultrasound technologies. The competitive landscape is expected to evolve further as vendors emphasize lifecycle management, interoperability, and AI-driven image interpretation as key differentiators.